Here, you will learn the test for different gases.
This level, you will learn different tests for 4 gases.
The four gases are Oxygen, Carbon Dioxide, Hydrogen and Ammonia.
Oxygen
To test whether oxygen is present, simply follow the theory of the Combustion Triangle. By lighting a glowing splint, the splint will relight/rekindle/reignite if oxygen is present. By using the catalyst ( Manganese ( IV ) Oxide ), it will speed up the chemical reaction and reignite the splint faster!
This is an equation for the test for Oxygen.
Hydrogen Peroxide —( Manganese ( IV ) Oxide )→ Oxygen + Water
Carbon Dioxide
Carbon Dioxide is the gas exhaled from our body. It's chemical name is CO2. It is produced by adding Carbon with Oxygen. To test whether Carbon Dioxide is present, simply bubble the limewater (Calcium Hydroxide). If there is presence of Carbon Dioxide, Limewater will turn from colorless to milky.
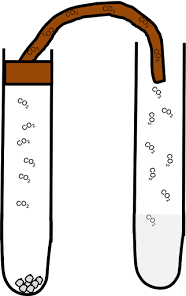 |
| Carbon Dioxide |
Hydrogen
Hydrogen test is quite similar to the test for oxygen, although some steps might be slightly different. By lighting a lighted splint, the fire on the splint will extinguish with a "pop" sound should there be hydrogen present.
Hydrogen, is said to be flammable, which has caused the ever famous Hindenburg Disaster, but extinguishes the fire quickly! This is because that Hydrogen is very flammable and when in contact with Oxygen, it causes a small explosion, similar to the incident to Hindenburg Disaster, which the Hydrogen exploded when contact with air. And because of this, it causes a "pop" sound.
As for Oxygen, because Oxygen has been used up in the chemical reaction, therefore, the fire extinguishes.
Ammonia
Ammonia gas, among all the four tests of gases, is the easiest to understand. Ammonia itself is an Alkali, a soluble base. An Alkali is a chemical compound that releases negatively charged hydroxide ions when dissolved in water. Only the Hydroxide Ions has most of the Alkaline properties, Therefore, a damp red litmus paper is used to test Ammonia.
Let's not forget the properties of Alkali, which turns Red Litmus paper to blue. And Ammonia is an Alkali. So, to test Ammonia, a damp red litmus paper is used. Damp is to release the negatively charged hydroxide ions, red litmus paper is to test whether Ammonia is present, which when the Litmus paper turns blue, it means that the statement of the presence of Ammonia is positive.
This is the end of Definitive Test for 4 gases.
To go back to Homepage, click this link :
Or
To go to Chemistry Element Page, click this link :



No comments:
Post a Comment